You
walk across a rug and reach for a doorknob. ZAP!! You get a shock.
Did you ever ask why? What is static electricity?
Everything we see is made up of tiny little parts called atoms. The atoms are made of even smaller parts. These are called protons, electrons and neutrons. They are very different from each other in many ways.
One way they are different is their "charge." Protons have a positive (+) charge. Electrons have a negative (-) charge. Neutrons have no charge.
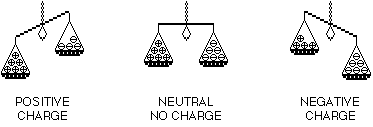
Usually, atoms have the same number of electrons and protons. Then the atom has no charge, it is "neutral."
But if you rub things together, electrons can move from one atom to another. Some atoms get extra electrons. They have a negative charge. Other atoms lose electrons. They have a positive charge. When charges are separated like this, it is called static electricity.
 If two things have the same charge, they repel,
or push away from each other.
If two things have the same charge, they repel,
or push away from each other.
If two things have different charges, they attract, or pull towards each other.
So, why does your hair stand up after you take your hat off?
When you pull your hat off, it rubs against your hair. Electrons move from your hair to the hat.
 Now each of the hairs has the same positive charge.
Things with the same charge repel each other. So the hairs try to
move away from each other. The farthest they can get is to stand up
and away from all the other hairs.
Now each of the hairs has the same positive charge.
Things with the same charge repel each other. So the hairs try to
move away from each other. The farthest they can get is to stand up
and away from all the other hairs.
If you walk across a carpet, electrons move from the rug to you. Now you have extra electrons. Touch a door knob and ZAP! The electrons move from you to the knob. You get a shock.
Imagine a pure gold ring. Divide it in half and give one of the halves away. Keep dividing and dividing and dividing. Soon you will have a piece so small you will not be able to see it without a microscope. It may be very, very small, but it is still a piece of gold.

If you could keep dividing it into smaller and smaller pieces, you would finally get to the smallest piece of gold possible. It is called an atom. If you divided it into smaller pieces, it would no longer be gold.
Everything around us is made of atoms and scientists so far know of 118 different kinds. These different kinds of atoms are called "elements." There are 98 elements that exist naturally (although some are only found in very small amounts). Four of these 118 elements have reportedly been discovered, but have not yet been confirmed.
Atoms join together in many different combinations to form molecules, and create all of the materials you see around you.
So what are atoms made of? In the middle of each atom is a "nucleus." The nucleus contains two kinds of tiny particles, called protons and neutrons. Orbiting around the nucleus are even smaller particles called electrons. The 115 kinds of atoms are different from each other because they have different numbers of protons, neutrons and electrons.
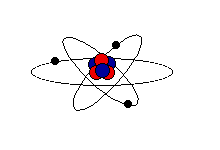
It is useful to think of a model of the atom as similar to the solar system. The nucleus is in the center of the atom, like the sun in the center of the solar system. The electrons orbit around the nucleus like the planets around the sun.
Just like in the solar system, the nucleus is large compared to the electrons. The atom is mostly empty space. And the electrons are very far away from the nucleus. While this model is not completely accurate, we can use it to help us understand static electricity.
(Note: A more accurate model would show the electrons moving in 3- dimensional volumes with different shapes, called orbitals. This may be discussed in a future issue.)
The charge of one proton is equal in strength to the charge of one electron. When the number of protons in an atom equals the number of electrons, the atom itself has no overall charge, it is neutral.
The protons and neutrons in the nucleus are held together very tightly. Normally the nucleus does not change. But some of the outer electrons are held very loosely. They can move from one atom to another.
An atom that loses electrons has more positive charges (protons) than negative charges (electrons). It is positively charged. An atom that gains electrons has more negative than positive particles. It has a negative charge. A charged atom is called an "ion."
Some materials hold their electrons very tightly. Electrons do not move through them very well. These things are called insulators. Plastic, cloth, glass and dry air are good insulators. Other materials have some loosely held electrons, which move through them very easily. These are called conductors. Most metals are good conductors.
How can we move electrons from one place to another? One very common way is to rub two objects together. If they are made of different materials, and are both insulators, electrons may be transferred (or moved) from one to the other. The more rubbing, the more electrons move, and the larger the static charge that builds up. (Scientists believe that it is not the rubbing or friction that causes electrons to move. It is simply the contact between two different materials. Rubbing just increases the contact area between them.)
Static electricity is the imbalance
of
positive and negative
charges.
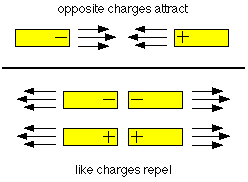 Now, positive and negative charges behave in
interesting ways. Did you ever hear the saying that opposites
attract? Well, it's true. Two things with opposite, or different
charges (a positive and a negative) will attract, or pull towards
each other. Things with the same charge (two positives or two
negatives) will repel, or push away from each other.
Now, positive and negative charges behave in
interesting ways. Did you ever hear the saying that opposites
attract? Well, it's true. Two things with opposite, or different
charges (a positive and a negative) will attract, or pull towards
each other. Things with the same charge (two positives or two
negatives) will repel, or push away from each other.
A charged object will also attract something that is neutral. Think about how you can make a balloon stick to the wall.
If you charge a balloon by rubbing it on your hair, it picks up extra electrons and has a negative charge. Holding it near a neutral object will make the charges in that object move.
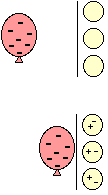 If it is a conductor, many electrons move
easily to the other side, as far from the balloon as
possible.
If it is a conductor, many electrons move
easily to the other side, as far from the balloon as
possible.
If it is an insulator, the electrons in the atoms and molecules can only move very slightly to one side, away from the balloon.
In either case, there are more positive charges closer to the negative balloon.
Opposites attract. The balloon sticks. (At least until the electrons on the balloon slowly leak off.) It works the same way for neutral and positively charged objects.
So what does all this have to do with static shocks? Or static electricity in hair?
When you take off your wool hat, it rubs against your hair. Electrons move from your hair to the hat. A static charge builds up and now each of the hairs has the same positive charge.
Remember, things with the same charge repel each other. So the hairs try to get as far from each other as possible. The farthest they can get is by standing up and away from the others. And that is how static electricity causes a bad hair day!
When we charge something with static electricity, no electrons are made or destroyed. No new protons appear or disappear. Electrons are just moved from one place to another. The net, or total, electric charge stays the same. This is called the principle of conservation of charge.
Charged objects create an invisible electric force field around themselves. The strength of this field depends on many things, including the amount of charge, distance involved, and shape of the objects. This can become very complicated. We can simplify things by working with "point sources" of charge. Point sources are charged objects which are much, much smaller than the distance between them.
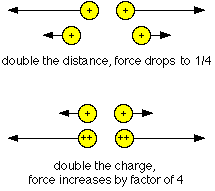 Charles Coulomb first described electric field
strengths in the 1780's. He found that for point charges, the
electrical force varies directly with the product of the charges.
In other words, the greater the charges, the stronger the field.
And the field varies inversely with the square of the distance
between the charges. This means that the greater the distance, the
weaker the force becomes. This can be written as the
formula:
Charles Coulomb first described electric field
strengths in the 1780's. He found that for point charges, the
electrical force varies directly with the product of the charges.
In other words, the greater the charges, the stronger the field.
And the field varies inversely with the square of the distance
between the charges. This means that the greater the distance, the
weaker the force becomes. This can be written as the
formula:
F = k (Q1 X Q2) / d2
where F is the force, Q1 and Q2 are the charges, and d is the distance between the charges. K is the proportionality constant, and depends on the material separating the charges.
What you need:
What to do:
What you need:
What to do:
What Happened: The neutral water was attracted to the charged comb, and moved towards it.
What you need:
SAFETY NOTE: DO NOT USE ELECTRICITY FROM A WALL OUTLET FOR THIS EXPERIMENT. Handle the glass light bulb with care to avoid breakage. The bulb can be wrapped in sticky, transparent tape to reduce the chance of injury if it does
What to do:
What Happened: When the charged comb touched the bulb, electrons moved from it to the bulb, causing the small sparks of light inside. In normal operation, the electrons to light the bulb come from the electrical power lines through a wire in the end of the tube.
This document summarizes safety issues related to static electricity, bonding and grounding containers, etc. when using flammable and combustible liquids. The scope of this document can be seen by checking the list of questions above. The documentFlammable and Combustible Liquids and their Hazards describes the hazards of these liquids more fully. Another document deals with the general safe work practices to follow where flammable and combustible liquids are used or stored.
Static electricity is the electric charge generated when there is friction between two things made of different materials or substances, like clothes tumbling in your dryer. Static electricity is what causes the sparks when you comb your hair or touch a metal object, like a doorknob, after walking across a carpet on a cold, dry day (especially during Canadian winters). It can also be generated by repeated contact and separation between unlike materials, like a flat belt on a rotating pulley.
Electric charges can build up on an object or liquid when certain liquids (e.g., petroleum solvents, fuels) move in contact with other materials. This can occur when liquids are poured, pumped, filtered, agitated, stirred or flow through pipes. This buildup of electrical charge is called static electricity. Even when liquids are transported or handled in non-conductive containers, something rubbing the outside surface of the container may cause a static charge to build up in the liquid. The amount of charge that develops depends, in part, on how much liquid is involved and how fast is it flowing or is being agitated or stirred.
Depending on circumstances it can be a nuisance or a hazard. Static cling in your clothes can be a nuisance but a spark that has enough energy to cause a fire or explosion is a definite hazard. To decide if static electricity is likely to be a hazard, you must consider several factors:
If the answer to the above five questions is yes where a solvent or fuel is used, then static electricity can be a fire / explosion hazard. It means that the spark can ignite a vapour/air mixture that is in its flammable range, the concentration range between the upper and the lower flammable limits.
Flammable and combustible liquids can present a static electricity hazard depending on their ability to generate static electricity, how well they conduct electricity (conductivity), and their flash point.
Solvents and fuels produced from petroleum (e.g., benzene, toluene, mineral spirits, gasoline, jet fuel) can build up a charge when they are poured or flow through hoses. They tend to hold a charge because they cannot conduct electricity well enough to discharge when in contact with a conducting material, like a metal pipe or container, that is grounded. When enough of a charge is built up, a spark may result. If the vapour concentration of the liquid in air is in the "flammable range" and the spark has enough energy, a fire or explosion can result.
According to the NFPA (Code 77), solvents that are soluble in water (or can dissolve some water themselves) do not build up static electricity. Examples of such liquids include alcohols and ketones like acetone. However, when liquids are transferred into non-conductive containers (e.g., plastic, glass), even conductive solvents may build up a charge because the plastic or glass containers decrease the rate at which the charge in the solvent dissipates.
The flash point and vapour pressure of the liquid and the temperature are other factors to consider. The vapour levels will be higher in the air around the container if you are working outside on a hot summer day than in the winter when the temperature is below 0°C (32°F) or colder.
At higher elevations in the mountains, the air pressure is significantly lower and solvents boil at lower temperatures. Under these conditions, the flash point and the temperature for the optimal vapour/air ratio are lower and some "combustible" liquids can become "flammable".
A liquid like hexane has a low flash point and it is flammable when its temperature is in the range -33°C to -3°C (-28°F to +26°F) at sea level. At normal room temperatures, the vapour/air ratio at the surface of the solvent will be well above its upper flammability limit and would be "too rich" to burn. However, at some distance away from the solvent surface, there is a concentration of hexane vapour in the air that is in the flammable range.
A fuel like kerosene is a combustible liquid with a flash point above 38°C (100°F). Under hot weather conditions or if high flash point liquids are heated to temperatures around or above their flash points, a flammable vapour/air mixture will form.
Generally, the conditions for igniting a liquid are optimal when the liquid is used at a temperature that produces a vapour in air concentration (at the surface of the liquid) that is halfway between the upper and lower flammability limits. Recognizing that these conditions represent an "optimal" fire hazard, one has to take appropriate precautions.
Transferring a liquid from one metal container to another may result in static electrical sparks. To prevent the build up of static electricity and prevent sparks from causing a fire, it is important to bond metal dispensing and receiving containers together before pouring. Bonding is done by making an electrical connection from one metal container to the other. This ensures that there will be no difference in electrical potential between the two containers and, therefore, no sparks will be formed.
The best way to bond containers is to securely attach a special metal bonding strap or wire to both containers. Some liquid transfer pumps have self-bonding hoses. Bonding can also be done by keeping a solid metal-to-metal contact between the containers themselves or between a metal container and a conducting nozzle. These latter two methods are usually not reliable because a good electrical contact is often hard to make and maintain during the entire transfer.
In the flammable liquid storage and dispensing area, ground dispensing drums. Grounding is done by connecting the container to an already grounded object that will conduct electricity. This could be a buried metal plate, a metallic underground gas piping system, metal water pipes or a grounded, metal building framework. Bonding both containers and grounding one of them "drains off" static charges and prevents the discharge of sparks. All grounding and bonding connections must be bare metal to bare metal. Remove all dirt, paint, rust or corrosion from points of contact. Specially designed and approved bonding and grounding wire assemblies are available from safety equipment retailers.
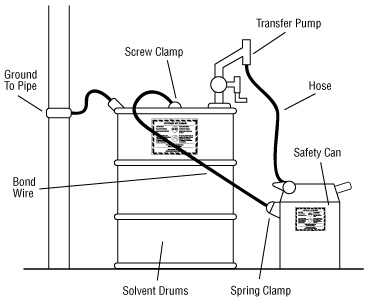
You only need to bond those containers that conduct electricity, such as those made from metal or special, conductive plastics.
If a container is made from a material that does not conduct electricity, such as polyethylene plastic or glass, bonding or grounding is not necessary: in fact grounding the container will not have any effect .
Even if a liquid is conductive, filling or handling plastic or other non-conducting containers can be hazardous. The splashing and turbulence of the liquid in the container can cause a static electric charge to build up in the liquid or on conductive parts on the container that are not grounded. A spark with enough energy to ignite a vapour/air mixture in its flammable range (an incendive discharge) can originate from the liquid or from the container.
For medium-sized containers (5 - 60 U.S. gallons or about 19 - 227 L) it is advisable to ground any metal parts on the container (and nearby conductive surfaces that the container may come in contact) and fill the container from the bottom through a long, grounded metal pipe. This procedure will reduce the amount of static charge produced and will enable the generated charge to relax (dissipate) through the metal pipe.
When filling non-conducting portable containers, the NFPA recommends that a grounded dip pipe or grounded wire be in the liquid in the container while it is being filled. The filling rate should be minimized, especially if there is filter in the line. Any metal parts of the container and metal funnel, if one is used, should also be grounded. When filling containers with low-conductivity liquids (i.e., ones with a conductivity less than 50 picoSiemens, pS), one should keep the grounded dip rod in the liquid for around 30 seconds after the filling is completed.
Similarly, filling an ungrounded portable fuel tank on a plastic-lined truck bed can cause spark-induced gasoline fires. For that reason, portable fuel tanks should be removed a safe distance from the vehicle (which, of course, is turned off) and be filled on the ground. The nozzle should be held in contact with the container while it is being filled.
Bonding and grounding are needed when dispensing flammable or hot combustible liquids from storage drums to smaller electrically conductive containers. Similarly, whenever you transfer these liquids between conductive containers in any work areas, for example, when filling or draining dip tanks, mixers, rinse tanks or other equipment, bond both containers together and ground one of them. Check bonding and grounding connections regularly to ensure they are in good condition.
Sri Neelakanteswara ITI